Analytical Method - ID50EAL ("IDEAL 50")
Standardized potency values were established by a skin test titration study (ID50EAL) using a limited number of patients. “ID50EAL” is an acronym for “Intradermal Dilution for 50 mm sum of Erythema determines the bioequivalent ALlergy units.” This study measured the size of the erythema in allergic patients receiving a sequence of 3-fold serially diluted extracts administered through intradermal injection. The dilution of extract that on average produced a 50 mm erythema (sum of length and width) was arbitrarily assigned a potency of “10,000 BAU/mL” if the dilution was between #11 and 13, or “100,000 BAU/mL” if the dilution was between #13 and 15.
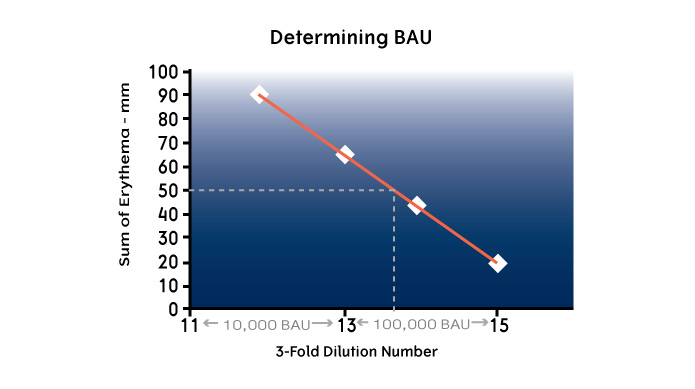
The ID50EAL method was only used once to determine which extract fulfilled these potency requirements. Since the original skin tests were done, representative extracts for each new in vitro reference lot are purchased by the FDA from one of the major extract manufacturers. In an evaluation period, all manufacturers test the new standard reference against the old to see if the results are consistent. When this reference is approved by the manufacturers, it is used as the standard to evaluate new lots of standardized extracts.


