Intradermal Dilutions
In early 2013, several allergen extract manufacturers decided to discontinue stock formulations of intradermal products. In place of this, it is recommended that you perform your own dilutions using the extract stock concentrations.
Rationale for the discontinuation of intradermal strength products
Allergen extracts that have been diluted to intradermal strength are known to lack the stability of concentrated solutions. Since intradermal strength extracts are manufactured as aqueous formulations, these products are prone to protein degradation that may impact their potency through the assigned expiration date.
Studies have demonstrated that using Human Serum Albumin (HSA) as a diluent increases the stability of extracts and can protect against protein degradation as compared with Normal Saline with Phenol (NSP).
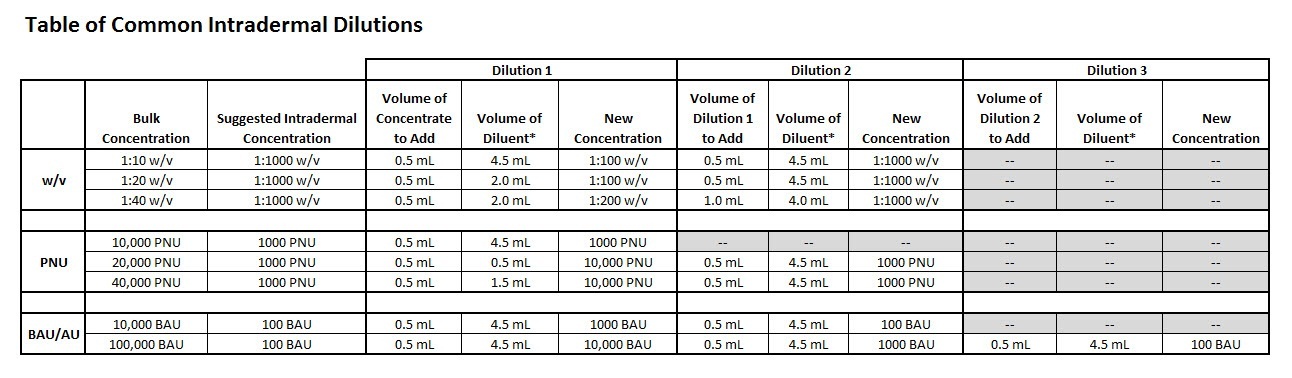
Prepare intradermal strength dilutions from the manufacturer’s concentrate
Many allergy practices prepare dilutions of concentrated allergen extracts for intradermal use. For your convenience, a table of common intradermal dilutions is provided that shows the steps required when preparing these concentrations in your practice.